LIMS are widely used during the development and manufacturing of FDA regulated products such as pharmaceuticals and medical devices. LIMS can play a major role in obtaining consistency, reliability, integrity, and accuracy of data, conforming with 21CFR Part 11 requirements. In fact, LIMS automation, when properly employed, can be a significant enabler to achieving GxP laboratory operation in regulated industries, eliminating tedious, manual, error-prone operations that are impossible to credibly validate and control. In these cases, computer system validation is essential in achieving the overall FDA mandated requirement that “equipment must be suitable for its intended use”. Although not mandated by the FDA, one model for validation that has gained international acceptance is represented in the diagram below, as sponsored by the GAMP (Good Automated Manufacturing Practices) Forum subcommittee of the ISPE.
The validation of COTS (Commercial Off The Shelf) software systems such as LIMS represents a special case of the GAMP model, and the validation is most effectively achieved by an appropriate mix of vendor and customer roles as suggested in this diagram.
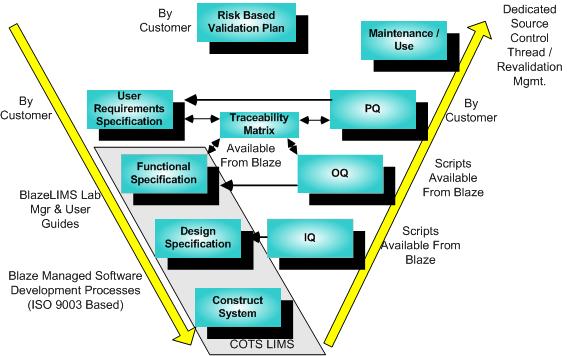
The validation process begins with the development of a Risk-Based Validation Plan by the Customer. It proceeds through the successive steps following the yellow arrows.
The validation process begins with the development of a Risk-Based Validation Plan by the Customer. It proceeds through the successive steps following the yellow arrows.
Blaze Systems can provide effective direction and assistance with any of the steps shown. Moreover, substantial cost savings over custom development can also be realized by taking advantage of several Blaze Systems product offerings. Given a User Requirements Specification for the specific installation and use, Blaze Systems can provide a Traceability Matrix which ties together the Functional and Design Specification embodied in the BlazeLIMS User Guides, and OQ validation scripts pared down to cover the elements of validation selected on a risk basis. This validation package does not merely provide generic validaton templates, but includes provisions for a complete validation environment, including consistent traceability references to Functional and Design Specs as embodied in LIMS documentation, automatic database initialization for test cases, “cherry picking” for effective risk based validation combined with system level feature blinding, and much more. The gains in savings and comprehensiveness, not only on test plan development, but also on test plan execution, can be dramatic, producing a defensible but least burdensome approach. Another high value offering critical to effective life cycle support provides a dedicated, rigidly managed source control thread and environment, so that bug fixes and enhancements may be performed and an associated impact statement and least cost regression validation plan executed. Finally, the BlazeDataMover provides a powerful automation tool to migrate configuration data between test, validation and production environments.
Benefits
- Least cost solution over green field validation efforts.
- Substantially reduced project duration.
- Reduced demands on lab staff.
- Bug fixes and enhancements managed via impact statement and suitable regression testing.
- Can employ optimal mix of customer / vendor / consultant resources based on each situation.
Features
- Integrated document package with consistent named linkages for traceability.
- Modular, integrated scripts provide superior ability to specify and execute validation scope based on risk analysis.
- Automated test case data loads provide comprehensive coverage and reduced execution time/labor.
- Lifetime dedicated source control thread for absolute change control and defensible regression testing.
- Readily augmented to cover customizations.
- Controlled blinding of unwanted/un-validated features.
- BlazeDataMover available to automate the migration of configuration changes from test to validation to production environments.
Taken together, these offerings can substantially reduce the life cycle costs of achieving and maintaining GxP laboratory operation.